| Adapted for the Internet from: Why God Doesn't Exist |
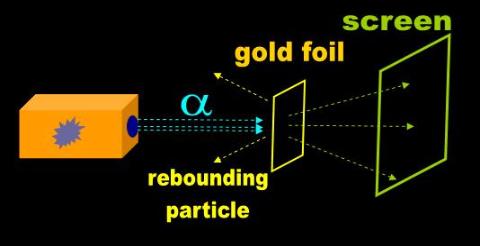
In 1911, Rutherford observed that radiated alpha particles rebound after striking a very thin gold foil (Fig. 1). He theorized
that the atom is comprised of a tiny, dense, positively charged nucleus surrounded by negative electrons that neutralize
atomic charge and suggested that the atom is mostly empty space. Rutherford was in effect converging on a planetary
model of the atom consisting of negative charges orbiting a positive core.
| A Bohring atom! |


| You see, my prodigal son, the H-atom is wasteful and reckless like you and like our beloved Earth. Our planet not only orbits the Sun, but also jumps back and forth from one orbital level to the next. |
| the prodigal quantum jump. |
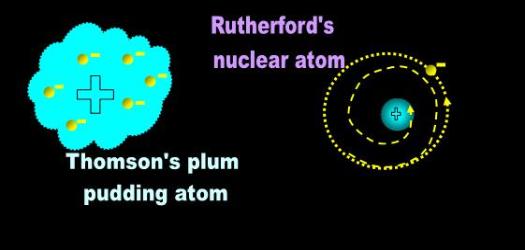
However, conservative British research circles of the times regarded Rutherford’s model with suspicion. Thomson, by
then a respected senior fellow, had proposed a ‘plum-pudding’ model of the atom consisting of a cloud of mass-less
positive charge sprinkled with dynamic negative charges (Fig. 2). [1] As always between the establishment and
revolutionaries, Thomson brought his authority to bear and won the day. He criticized Rutherford’s atom primarily on
grounds of instability, arguing that the orbiting electron would lose energy and spiral inwards and fall into the nucleus.
Contemporary mechanics synthesize Thomson’s lesson for us:
- “ if Newtonian mechanics governed the workings of an atom, electrons would rapidly
travel towards and collide with the nucleus.” [2]
Fig. 1 Rutherford's proton |
Most alpha particles run right through the foil, but some are kicked back as if they struck a brick wall. Rutherford theorized that this could only happen if the rebounding particles came in contact with an impenetrable region (the nucleus) of the atom. He surmised that the atom was mostly empty space, and was later able to estimate the diameter of the nucleus, which he dubbed the ‘proton.’ 52 Such interpretations led the way to the planetary model of the hydrogen atom. |
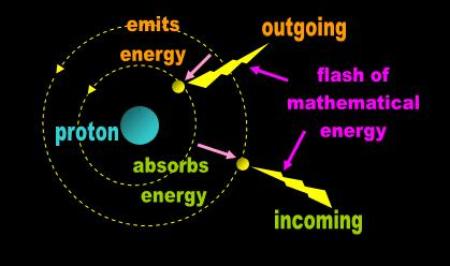
Bohr tipped the scales in Rutherford’s direction in 1913 when he proposed that the electron radiates and absorbs energy
in fixed quanta as it jumps back and forth between ‘orbitals’ (Fig. 3). This mathematical model purportedly addressed
Thomson’s stability concerns and explained Balmer spectral lines.
Fig. 2 Thomson’s plum pudding and Rutherford’s nuclear models of the atom |
| Thomson rejected Rutherford’s nuclear atom arguing that the orbiting negative electron should lose energy and spiral into the nucleus. |
- The result is that, today, QM has three models for the hydrogen atom, and the mathematicians use all three simultaneously.
They point to Bohr’s planetary system (Earth and orbiting Moon), but tell you that this model is only used to teach beginners.
- " The Bohr model is a primitive model of the hydrogen atom... and thus may be
considered to be an obsolete scientific theory... the Bohr model is still commonly
taught to introduce students to quantum mechanics, before moving on to the
more accurate but more complex valence shell atom." [3] [4]
- The mechanic boasts that they learn the high-level, cloud version of the atom in college:
- " Electron cloud is a term used for introducing the concept of wavefunction in
low-level pedagogical introductions to atomic physics, molecular physics,
chemistry or quantum chemistry. This idea corresponds to delocated electrons
moving or standing like clouds around the atomic or molecular nuclei. This is
indeed a better image than the very common image provided by the Bohr model
which commonly leads to a visualisation of electrons driving around the nuclei
along orbits like the planets around the sun." [5]
" According to the Copenhagen interpretation of quantum mechanics, a particular
electron is both ‘nowhere at all’ and ‘everywhere all at once’ until an act of
measurement causes it to be detected." [6]
" Protons and neutrons make up a dense, massive atomic nucleus, and are
collectively called nucleons. The electrons form the much larger electron cloud
surrounding the nucleus." [7]
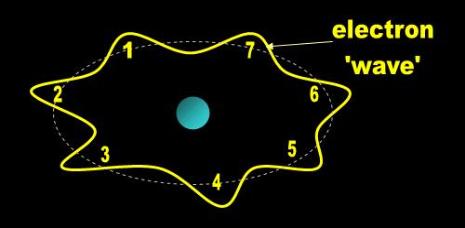
In addition to the planetary and cloud models, the mechanics sometimes bring up DeBroglie’s integral wave model (Fig. 4),
but only to show you that an electron can somehow be visualized as spread around the nucleus. Then they throw this
ribbon in the trash can for the rest of the presentation. The only model that the mechanics have formally rejected and use
exclusively for their History classes is Thomson’s plum-pudding atom.
Fig. 3 Bohr’s quantum jump |
| Bohr theorized that when the electron falls to a lower orbit, it emits energy, and when it rises to a higher one, it gains energy. Bohr’s theory addressed Thomson’ s concerns about stability and transformed Rutherford’s nuclear atom into a model that physicists could easily relate to. What is curious about Bohr’s explanation is that it is diametrical to the results of the Harvard Tower Experiment (HTE). The authors of the HTE paper argued that light leaving the Earth loses energy to the gravitational field and gains it when approaching. Bohr’s atom does it in reverse. Light departing an atom reduces the ‘energy’ of the atom’s ‘field’. . |
- However, these three models (wave, planetary, and cloud) are structurally so different that you wonder what an atom really
looks like (Fig. 5)? What are the mechanics talking about?
Fig. 4 DeBroglie’s Electron Waves |
| The electron doesn’t fall towards the nucleus as Thomson predicted because it also behaves like a wave. This electron has an integral number of waves and can occupy only a certain region around the nucleus. This model also simultaneously accounts for Bohr’s quantum jump and Thomson’s stability concerns. |
The scientific method absolutely demands that a proponent decide in advance whether the single, S-orbital electron of
hydrogen is a cloud, a shell, a ring, or a bead and then to use this hypothesis consistently throughout the dissertation.
The mechanics can’t do it this way. If they present a particle, they can’t explain why this discrete entity is smeared around
the atom or how this discrete entity keeps two atoms bound in a molecule. Therefore, they do the whole process in reverse.
They talk to you about their fantastic theories and about how the scientific community is smashing protons and electrons
to discover yet more particles and not once have they ever attempted to tell you what these particles look like. What QM is
missing is a valid hypothesis. A particle is a valid hypothesis, but then the mathematician must clarify how the shape of a
muon differs from an electron and explain why a muon lives 2 microseconds whereas an electron lives forever. To say
that a muon and an electron are just particles that have different weights doesn’t tell us anything. Is a muon bigger than
an electron in size? Will a mechanic put his life on the line for his answer?
It turns out that the mathematicians give lip service to DeBroglie’s wave and to Born’s cloud. These models are simply
used to make sense of DeBroglie and Schroedinger wave equations. The mechanics have no practical use for these models
any more than they have use for Thomson’s plum-pudding model. That’s all you’ll ever hear about them.
Despite wholesale denials and disclaimers the architecture used in Quantum Mechanics is still Bohr’s debunked planetary
model. The American Heritage Dictionary (AHD) defines an ion as an atom that has gained or lost one or more discrete
electron beads. [8] Ridley (pp. 18-19) defines electric current as the flow of discrete electron particles. [9] Davies (Ch. 4)
portrays scattering as the exchange of supernatural virtual photons between two discrete electron marbles. [10] And
Ebbing depicts covalent bonding as the inter-atomic sharing of discrete electron golf balls. [11] Indeed, discrete electrons
underlie Lewis’s shell theory.Schrödinger lent credibility to de Broglie´s Saturnian hypothesis, but the matter-wave
equation relies on discrete quantities for electron mass and charge, implying that a finite, discrete object underlies it
nevertheless. And Born’s (pp. 634-663) electron cloud is really a cloud of probability, the region around the nucleus
where a discrete electron bead is likely to be found (See Boslough).
The mechanics would prefer to forget that this is the official doctrine of the Church of Quantum, yet they peddle the
cloud as a physical balloon made of electron beads:
- “ The electron cloud is far larger than the size of the individual electron(s) which
comprise(s) it.” [12]
Therefore, the hydrogen atom in use today continues to be the Ptolemaic anachronism conjured by the Fathers of Quantum.
The integral wave, the shell, and the cloud are not architectural models of a physical electron, but regions occupied by
electron beads.
The experts who flaunt their knowledge at the Wikipedia illustrate the cloud model of the helium atom, indicating that this
is the model of their choice. [13] What they show is a bunch of dots extending radially from a center and this tends to
mislead people, who erroneously infer that particle mathematicians know what an atom looks like. The experts tell us
that these clouds represent regions where an electron bead is to be found:
- “ In the true modern model of the atom, the positions of the electrons around the
atom’s nucleus are described through probabilities – that is, an electron can
theoretically be found at any arbitrary position around the nucleus…This
pattern is referred to as its atomic orbital” [14]
- “ The spatial components of these one-electron functions are called atomic
orbitals…an atomic orbital is the region in which an electron may be found
around a single atom… Fundamentally, an atomic orbital is a one-electron
wavefunction… [15]
“ According to the Principles of Quantum Mechanics electrons are distributed
around the nucleus in ‘probability regions’. These probability regions are
called ‘atomic orbitals’. According to Quantum Mechanics, these orbitals are
mathematically defined… Each electron has a probability region which defines
the probability of finding that electron in a certain three dimensional space
around the nucleus.” [16]
- The problem comes when these clouds later interact with ‘regions’ of other atoms to form molecules:
- “ The nucleus of an atom is surrounded by a cloud of electrons, and it is
primarily the interaction of these clouds that govern the chemical behavior
of atoms…[17]
“ What happens when a covalent bond is formed between two fluorine atoms
is that an orbital from one atom overlaps in space with one from another atom.” [18]
“ electrons fill atomic orbitals in atoms…When two atomic orbitals overlap, they
interact in two extreme ways to form two molecular orbitals” [19]
So let’s do a sanity check on the mechanics’ claims. An atomic orbital is a region. Two or more of these regions overlap and
form a molecule. This is like saying that the aggregate of Pluto’s orbits over millions of years form an orbital. The mechanics
are saying that this abstract orbital physically interferes with Neptune’s orbital. The mathematicians have to be kidding if they
think this cloud model clarifies how two atoms physically interact! The sages of Mathematics are saying that a mathematical
function physically interacts with another function. They have the number 5 interacting with the number 6. That’s exactly what
the idiots of QM are saying. According to QM, the orbital is not a physical entity like a balloon or a basket or a cage. An orbital
is a region of space where an irrelevant electron bead was two hours ago. I say irrelevant because atomic bonding theory has
absolutely no use for an electron bead. The mechanics explain atomic bonding with orbitals. In QM, a mathematical probability
function comes to life as a 3-D object and interacts with another function. Molecular orbital theory is founded on the interaction
of abstract concepts.
Actually, the mathematicians who invented this nonsense did not really have in mind a physical cloud or shell. The ‘cloud’
model of the electron is a surrealistic physical interpretation that Born and others gave to Schrödinger’s wave equation.
QM’s famous ‘cloud’ is really a bunch of itineraries that surround a nucleus. Think of Mighty Mouse’s contrail with which
he lassos and ties up some mean cats. The QM cloud is likewise made of vapor trails, of locations that a bead occupied in
the past. The ridiculous cloud of Quantum Mechanics is not a photograph, but a movie of a bead that orbited a bowling ball
two hours ago (Fig 3.30). In other words, the wave equation does not show what the surface of an electron looks like. It
shows the trajectories of an electron bead. The morons of QM call this ‘thing’ a cloud (Born) or a shell (Lewis) or an orbital.
Then they postulate that these ‘orbits’ interact with each other to form molecules. Again, if ever the idiots of Mathematics
take a magnified snap shot of an H-atom, it had better not look like what the people at the Wikipedia illustrated because
this would instantly falsify Quantum Mechanics.
The punch line is that the mechanics propose that atoms rotate. [20] [21] This means that the mathematician first has to
wait until the electron bead travels and makes an orbital. Later, when a mechanic spins an atom, he is implicitly spinning
its orbital. Like relativists playing with their toy tesseracts, the mechanics end up moving motion itself?
Fig. 5 ‘The’ Quantum H atom |
| The mechanics use three different physical models of the H-atom to explain their theories and equations. De Bro-glie’s model is an inte-gral wave stretched around a ball. Bohr’s planetary system con-sists of a single bead going around in circles for no reason that anyone can explain. And Born’s proposal is really a region where you are most likely to find an electron bead (i.e., a probability). The mechanics openly admit that they have abandoned attempting to visualize what they are talking about, yet they also claim that they have photographed atoms and that their mathematical theory is a complete, accurate, and perfect. In science, it works in reverse. First you must point to the physical object and name it. Then you can explain anything you want with it. The mathematicians are effectively amending their assumptions retroactively. Therefore, it is self-aggrandizing to claim that Quantum is accurate and complete until the idiots of Mathematics resolve these fundamental discrepancies. If an electron is not a discrete particle, these models together with all of Quantum Mechanics instantly wind up in the ash heap of history. . |

- Module main page: They don't call it Quantum Magic for nothing!
This page: The Quantum electron: a bead, a ribbon, AND a cloud all in one!
Pages in this module:
1. Niels Bohr's ridiculous bead (1911)
2. De Broglie's more ridiculous ribbon (1923)
3. Max Born's even more ridiculous cloud (1926)
4. James's Bonds: A day at the York Chemistry Lab (2008)
5. Cramer believes he has seen an atom
- ________________________________________________________________________________________
- Copyright © by Nila Gaede 2008
